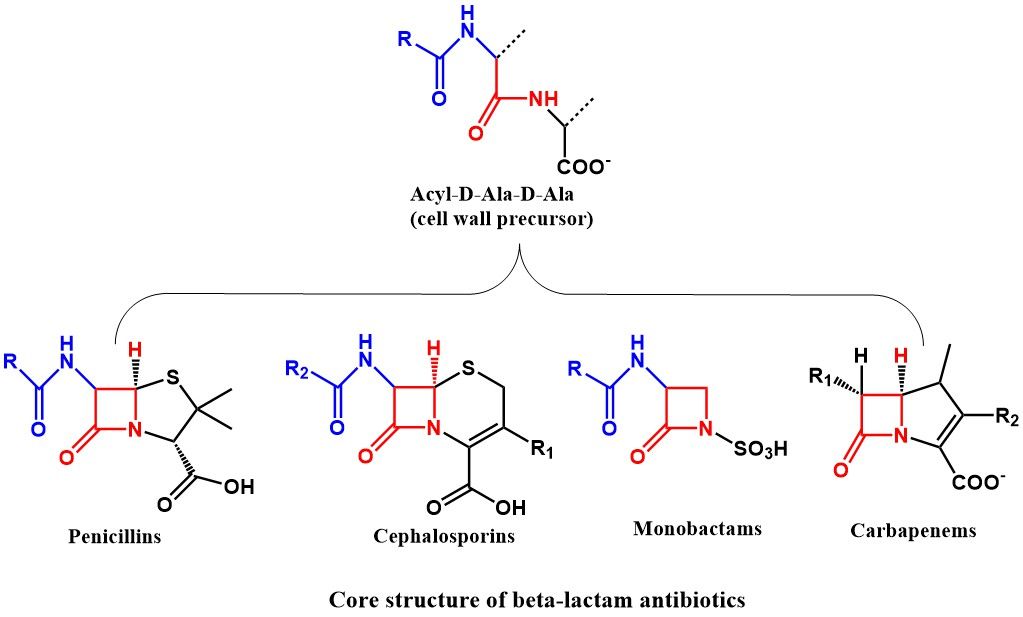
Frontiers | The Chemical Relationship Among Beta-Lactam Antibiotics and Potential Impacts on Reactivity and Decomposition
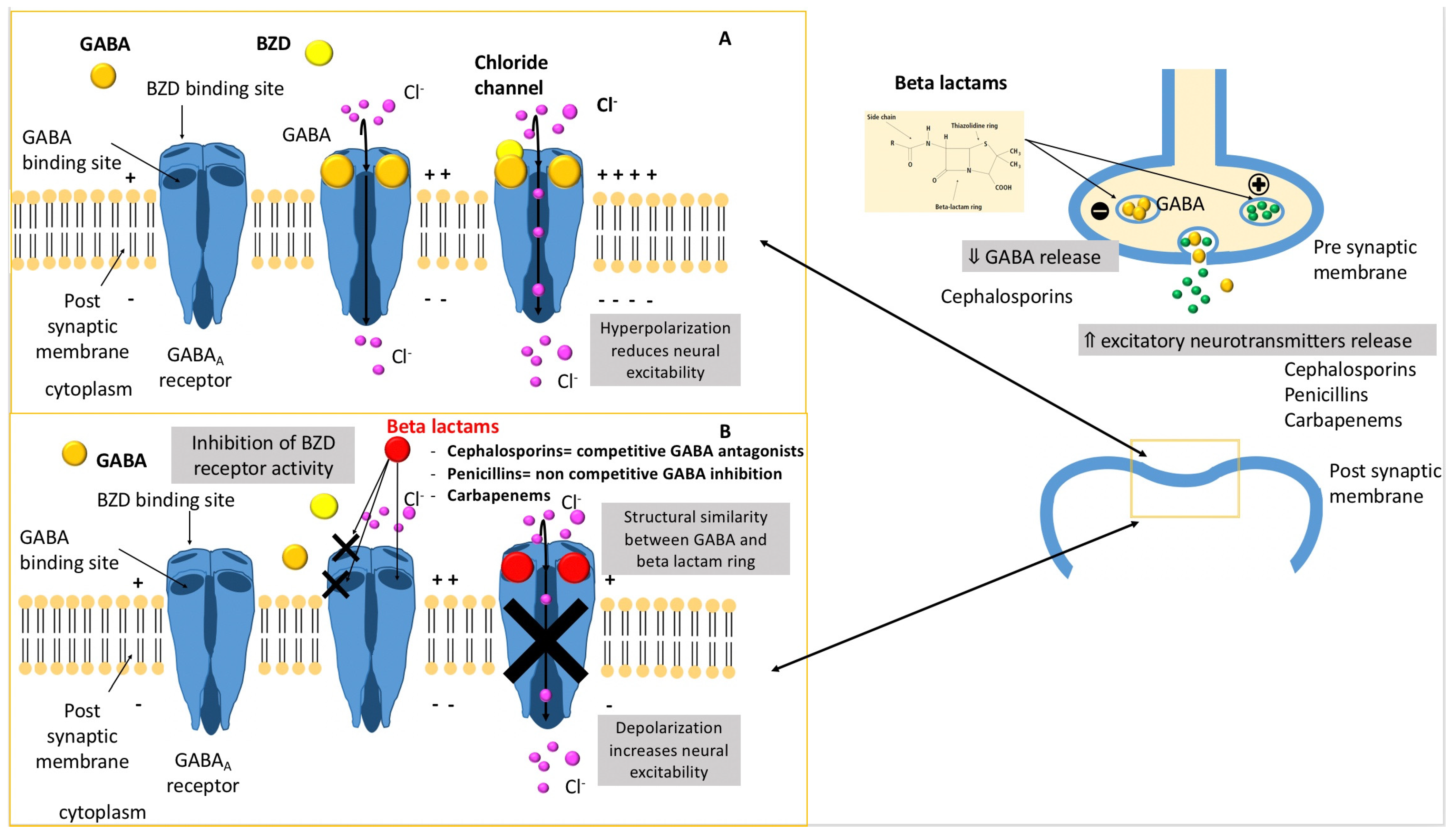
Microorganisms | Free Full-Text | Beta-Lactams Toxicity in the Intensive Care Unit: An Underestimated Collateral Damage?
The cleavage of the β-lactam ring by the β-lactamases. a) The β-lactam... | Download Scientific Diagram

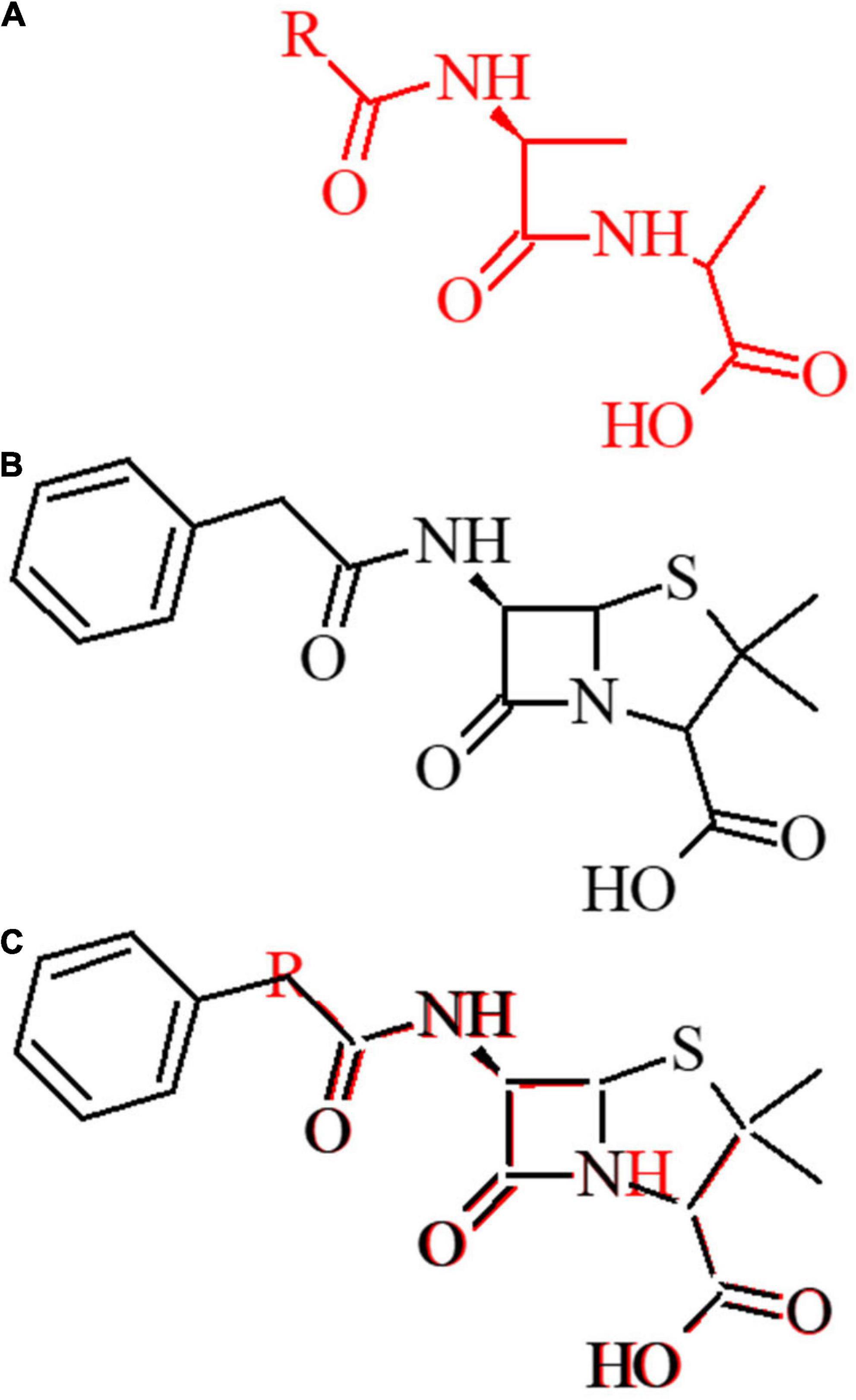
Synthetic Approaches toward Monocyclic 3‐Amino‐β‐lactams - Deketelaere - 2017 - ChemistryOpen - Wiley Online Library
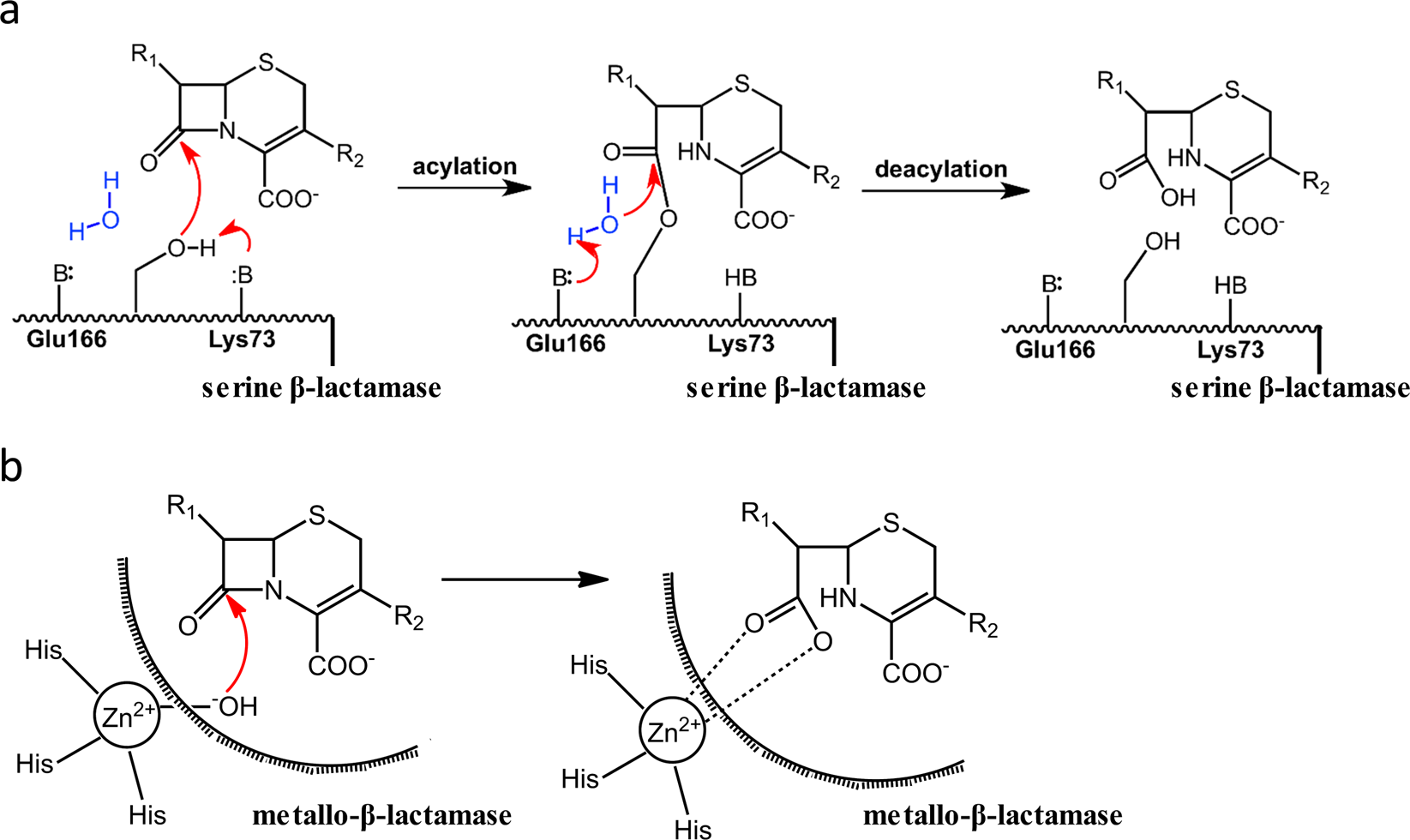
The hydrolytic water molecule of Class A β-lactamase relies on the acyl-enzyme intermediate ES* for proper coordination and catalysis | Scientific Reports
Antibiotics | Free Full-Text | Molecular Targets of β-Lactam-Based Antimicrobials: Beyond the Usual Suspects

Cysteine Hydropersulfide Inactivates β-Lactam Antibiotics with Formation of Ring-Opened Carbothioic S-Acids in Bacteria | ACS Chemical Biology
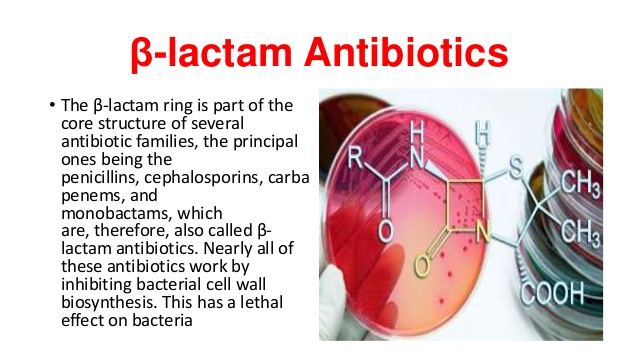

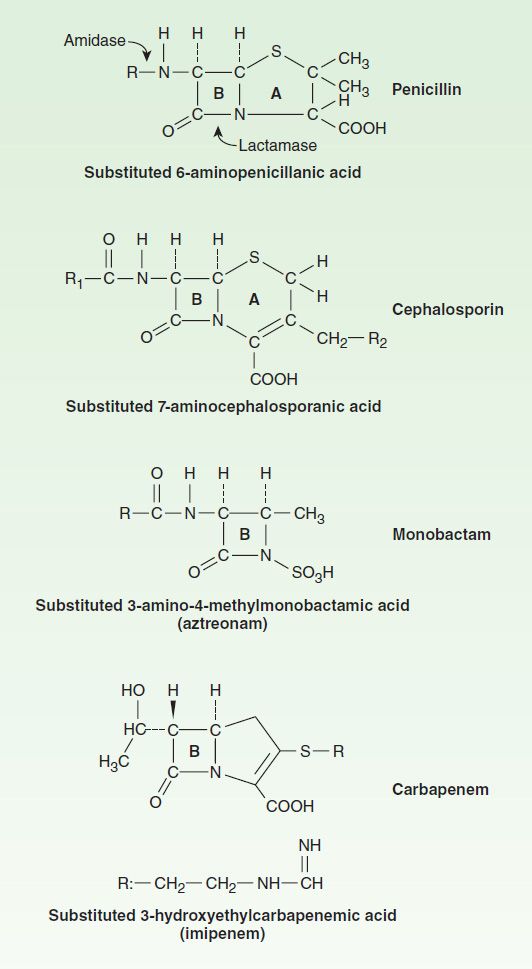


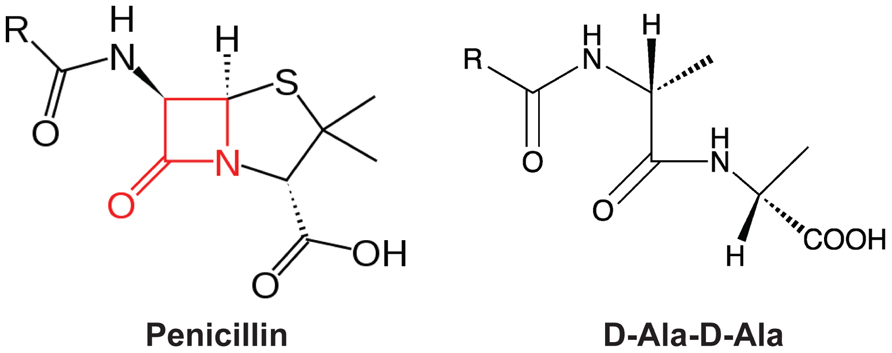

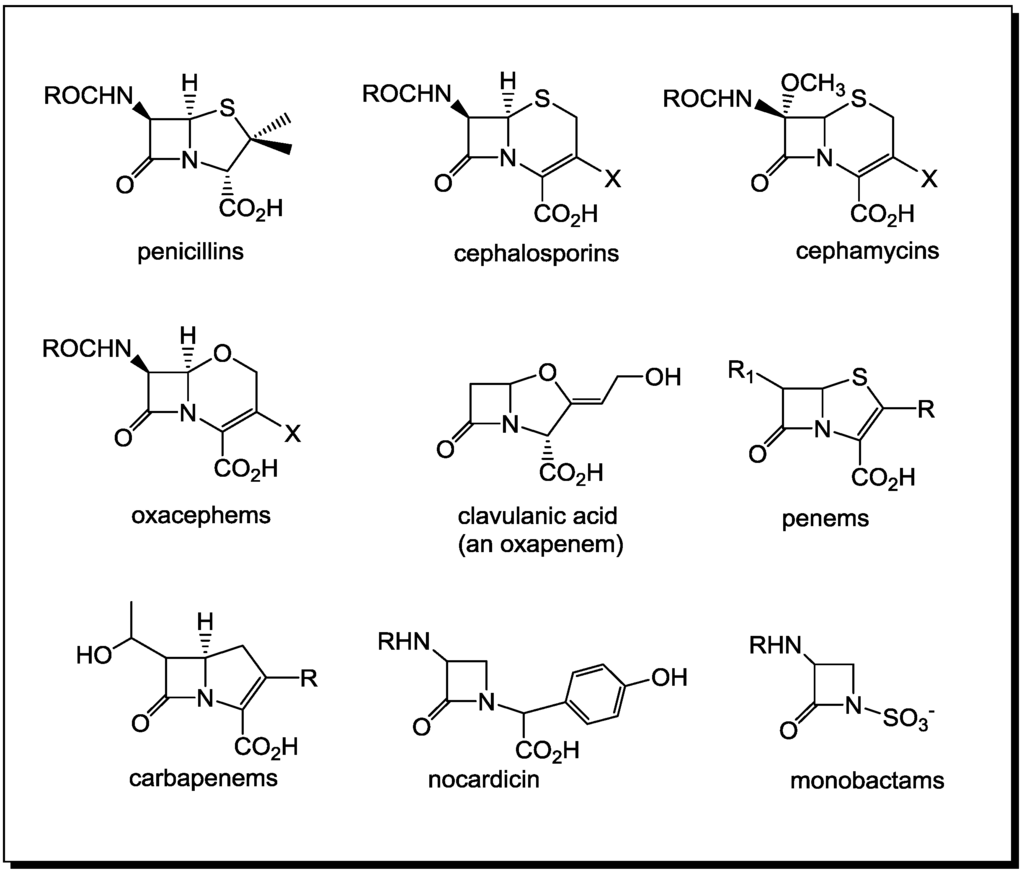
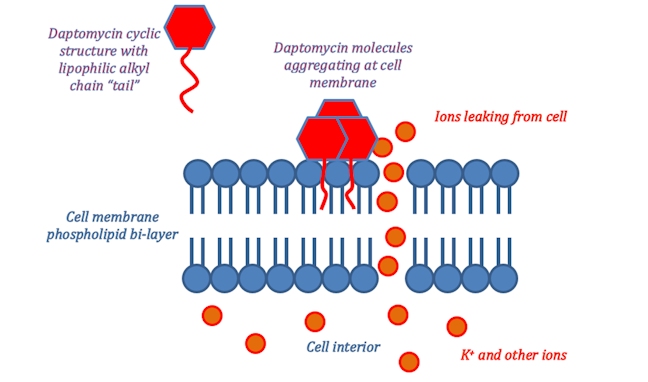
![betalactam_pharm [TUSOM | Pharmwiki] betalactam_pharm [TUSOM | Pharmwiki]](https://tmedweb.tulane.edu/pharmwiki/lib/exe/fetch.php/beta_lactam_moa2.png?w=700&tok=f5fe9e)
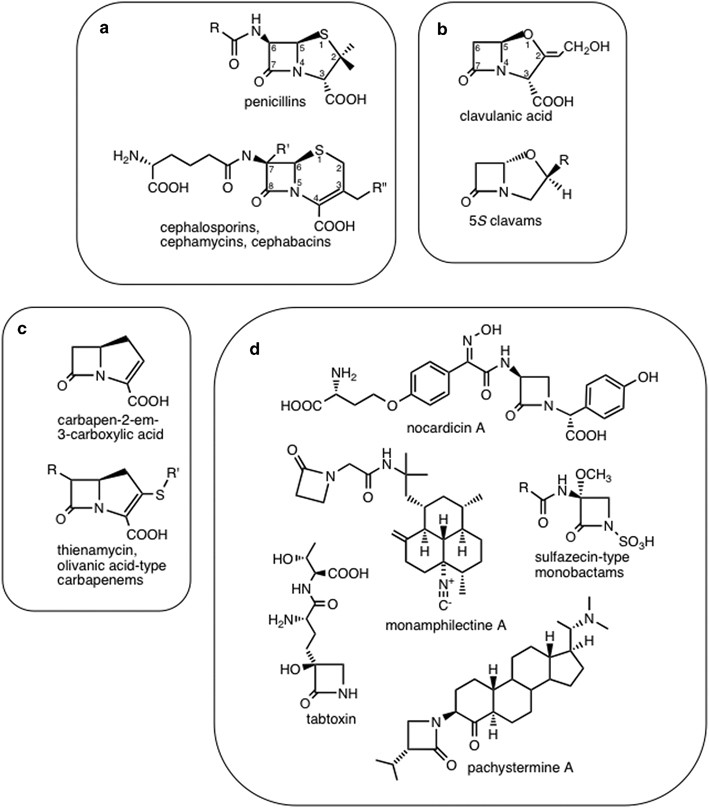
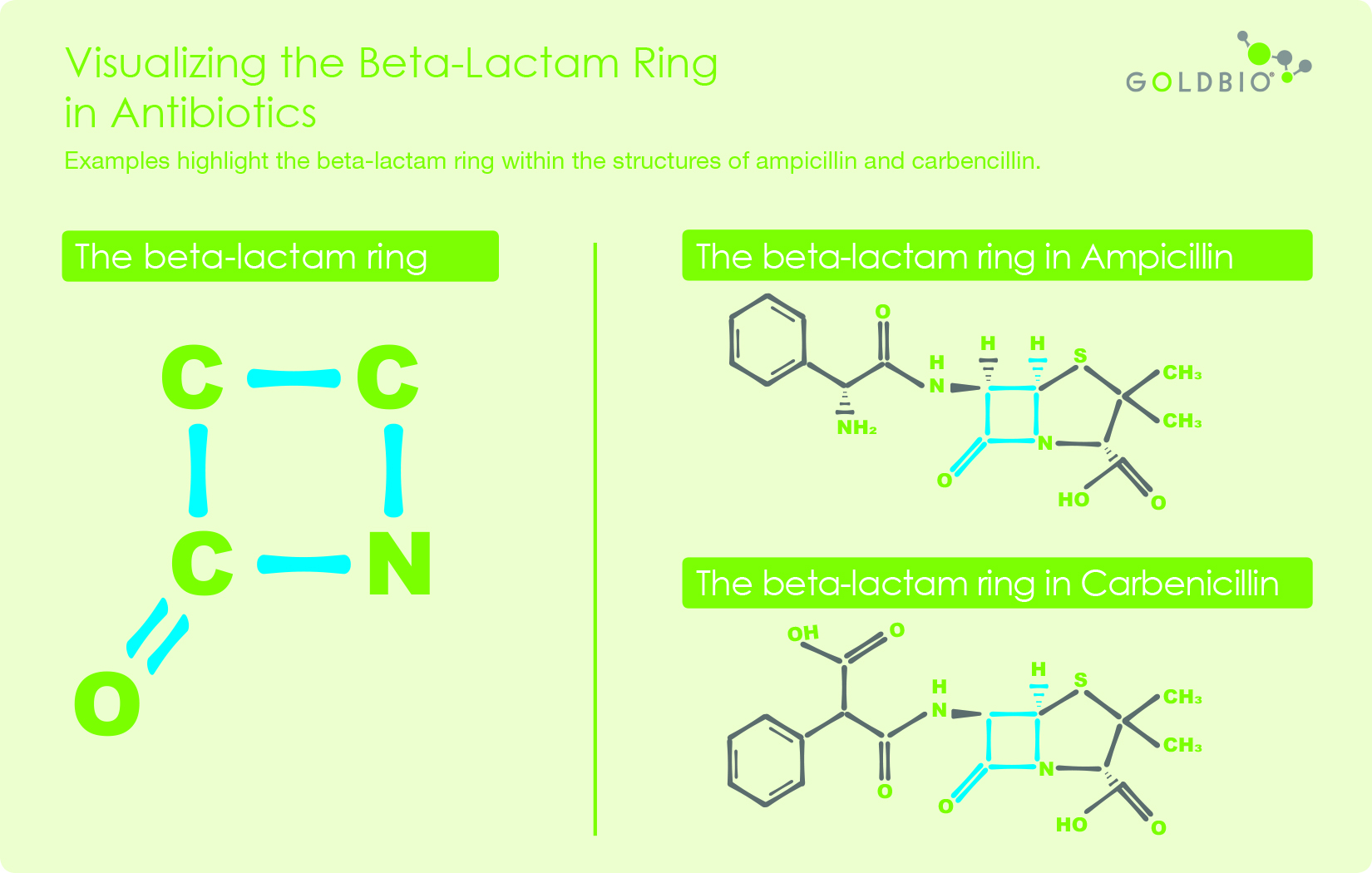
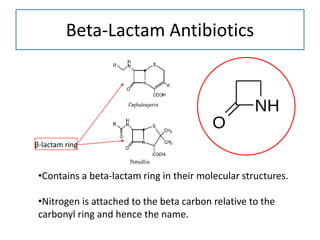

![betalactam_pharm [TUSOM | Pharmwiki] betalactam_pharm [TUSOM | Pharmwiki]](https://tmedweb.tulane.edu/pharmwiki/lib/exe/fetch.php/betalactam_structure.png?w=600&tok=54cff1)
![betalactam_pharm [TUSOM | Pharmwiki] betalactam_pharm [TUSOM | Pharmwiki]](https://tmedweb.tulane.edu/pharmwiki/lib/exe/fetch.php/betalactams.png?w=600&tok=3379e1)